Chemical Oxygen Demand Pdf
Dec 30, 2018 VoTT: Visual Object Tagging Tool 1.5. This tool provides end to end support for generating datasets and validating object detection models from video and image assets. The tool supports the following features: The ability to tag and annotate Image Directories or Stand alone videos. Apr 03, 2019 If you mean the tool that was part of the Picture and Fax Viewer, this program is no longer available in Windows 7. In Windows 7, you can use Paint to annotate images. Open your image Windows Paint and select the Text Tool (The icon with the capital A) This will reveal the toolbar with all of the options. Let us know if you have more questions.  Apr 03, 2014 LEADTOOLS Annotations. Implement hyperlinks for every object. Popular uses for hyperlinks include user defined messages, programs to run, or jumps to Web pages. Perform the following operations on a single object or group of objects (Scale, Translate, Rotate, Flip, Reverse).
Apr 03, 2014 LEADTOOLS Annotations. Implement hyperlinks for every object. Popular uses for hyperlinks include user defined messages, programs to run, or jumps to Web pages. Perform the following operations on a single object or group of objects (Scale, Translate, Rotate, Flip, Reverse).
Biochemical Oxygen Demand And Chemical Oxygen Demand
Chemical Oxygen Demand merupakan parameter kualitas air yang penting karena, mirip dengan BOD, ia dapat menilai dampak effluen air limbah yang akan dibuang pada ling kungan penerima (badan air). Tingkat COD tinggi menandakan banyaknya jumlah bahan organik yang teroksidasi pada sampel, yang akan mengurangi tingkat oksigen terlarut (DO). Chemical oxygen demand test uses a strong chemical oxidant in an acid solution and heat to oxidize organic carbon to CO2 and H2O. It can be expressed in milligrams per liter (mg/L) also referred to part per million (ppm) which indicates the mass of oxygen consumed per liter solution. The Chemistry of Chemical Oxygen Demand. By John Stone. Chemical Oxygen Demand (COD) is a quick, inexpensive means to determine organics in water. COD samples are prepared with a closed-reflux digestion followed by analysis. 5220 CHEMICAL OXYGEN DEMAND (COD).#(102) 5220 A. Introduction Chemical oxygen demand (COD) is defined as the amount of a specified oxidant that reacts with the sample under controlled conditions. The quantity of oxidant consumed is expressed in terms of its oxygen equivalence. Because of its unique chemical properties, the dichromate ion.
OXYGEN DEMAND
- required for oxidation of inorganic and organic matter.
- essential for the livelihood of micro organisms.
- can be measured by
- BOD – Biochemical oxygen demand
- COD – Chemical oxygen demand
Biochemical Oxygen Demand And Chemical Oxygen Demand
Biochemical Oxygen Demand
Introduction
- measures the quantity of oxygen consumed by microorganisms during the decomposition of organic matter.
- indirect measure of biodegradable organic compounds in water.
Significance
- determining degree of H2O pollution.
- Important measurement in operation of sewage treatment plant.
- Comparing BOD of incoming sewage & effluent- efficiency, effectiveness of treatment is judged.
- For example, in a typical residential city raw sewage has a BOD value of around 300 mg/L. If the effluent from the sewage treatment plant has a BOD. of about 30 mg/L, the plant has removed 90 percent of the BOD
Chemical Oxygen Demand Testing
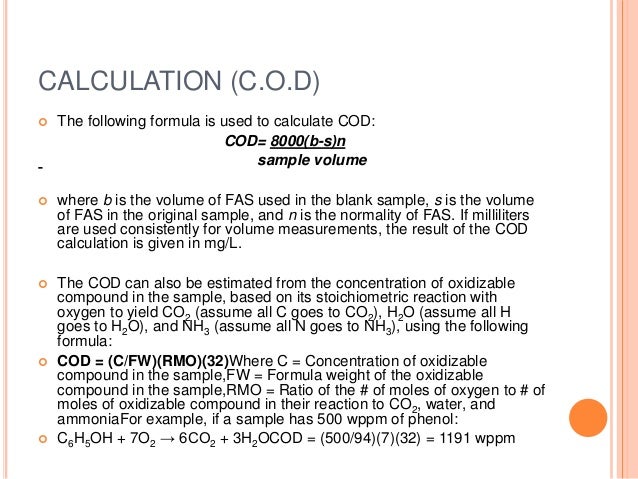
Chemical Oxygen Demand Lab Report
Dilution Method
- DO Is measured prior to incubation.
- allowed to stand for five days at a controlled temperature of 20 °C (68 °F).
- At the end of the five-day period, the remaining dissolved oxygen is measured.

- BODt = (DOi – DOf) × D.F.
Where,
Install the game setup completely. Halo pc free. After downloading open Daemon Tools and Mount Image the CD. Copy all content from “” Folder. After installation open CD Folder and then open “” Folder. When you will Mount Image the CD, setup will be launched.
- BODt = Biochemical oxygen demand at t days.
- DOi = initial dissolved oxygen before incubation.
- DOf = final dissolved oxygen.
- D.F.= dilution factor= volume of the bottle/volume of the sample.
Kinetics
Limitations
- Dilution is required.
- Pretreatment if toxic wastes.
- Long period of time.
- Seeding for industrial water.
Chemical Oxygen Demand
Introduction
- Measure of oxygen equivalent of the organic matter content of the sample that is susceptible to oxidation by a strong chemical oxidant (acid + heat).
- COD test results are used for monitoring and control of discharges, and for assessing treatment plant performance.
- Expressed in mg/l or ppm.
COD TEST
- Potassium dichromate, a strong oxidizing agent is used under acidic conditions to find the amount of organic compound in waste water sample.
- Acidity is usually achieved by the addition of sulphuric acid.
- The amount of Cr3+ is determined after oxidization is complete, and is used as an indirect measure of the organic contents of the water sample.
- To do so, the excess potassium dichromate is titrated with ferrous ammonium sulphate (FAS) until all of the excess oxidizing agent has been reduced to Cr3+.
Accuracy Of The Test
- It is important that no outside organic material be accidentally added to the sample to be measured.
- To control for this, blank sample is used created by adding all reagents (e.g. acid and oxidizing agent) to a volume of distilled water. COD is measured for both and the two are compared i.e. COD of blank – COD of sample.
Limitations
Chemical Oxygen Demand Test
- Chemical Oxidant is not specific to oxygen-consuming chemicals that are organic or inorganic, both of these sources of oxygen demand are measured in a COD assay.
- It does not distinguish between Biodegradable and Non-Biodegradable organic matter.
- The test does not measure the oxygen demand caused. by the oxidation of ammonia into nitrate
COD vs BOD
- Faster process control.
- COD and BOD do not necessarily measure the same types of oxygen consumption.
- COD is always greater than BOD measurements.
- COD is a more stable measurement method.
- How can a correlation be determined?
- Collect empirical data
- COD and BOD data for the same water sample collected over the same period of time.
- Graph data
- Graph COD and BOD data to determine whether or not a correlation exists.
Related Articles
- Laitinen, G.A. and Harris, W.E., Chemical Analysis, New York; McGraw-Hill, 1975, 2nd ed.Google Scholar
- Leithe, W., Die Analyse der organischen Verunreinigungen in Trink-, Brauch-, und Abwassern, Stuttgart: Wissenschaftliche, 1972.Google Scholar
- Lur’e, Yu.Yu., Analiticheskaya khimiya promyshlennykh stochnykh vod (Analytical Chemistry of Industrial Wastewater), Moscow: Khimiya, 1984.Google Scholar
- Posylaiko, V.I., Kozyreva, N.A., and Logacheva, Yu.P., Khimicheskie metody analiza (Chemical Methods of Analysis), Moscow: Vysshaya Shkola, 1989.Google Scholar
- Golovatyi, E.I., Instrumental’nye metody kontrolya BPK stochnykh vod mikrobiologicheskoi promyshlennosti: Obzornaya informatsiya (Instrumental Methods for Monitoring BOD of Wastewater from Microbiological Industries: Review), Moscow: ONTITEI Mikrobioprom, 1982.Google Scholar
- SO 6060-86: Quality of Water: Determination of Chemical Oxygen Demand.Google Scholar
- RF Patent 1 223 123.Google Scholar
- Dugin, G.V., Pisarevsky, A.M., and Polozova, I.P., Khim. Tekhnol. Vody, 1985, vol. 5, no.4, pp. 51–53.Google Scholar
- Dugin, G.V., Pisarevsky, A.M., Polozova, I.P., and Shul’ts, M.M., Zh. Prikl. Khim., 1986, vol. 59, no.1, pp. 22–27.Google Scholar
- Beliustin, A.A., Pisarevsky, A.M., Lepnev, G.P., et al., Sensors Actuators B, 1992, vol. 10, pp. 61–66.Google Scholar
- Kolichestvennyi khimicheskii analiz vod: Metodika vypolneniya izmerenii massovoi kontsentratsii khimicheski potreblyaemogo kisloroda v probakh prirodnykh i stochnykh vod bikhromatopotentsiometricheskim metodom. Minprirody Rossii (Quantitative Chemical Analysis of Water: Procedure for Determining Chemical Oxygen Demand (Mass Concentration) in Samples of Natural and Waste Water by Bichromatopotentiometric Method, Ministry of Environment of the Russian Federation), PND F 14.1:2.19-95, Moscow, 1995.Google Scholar
- Antsyshkina, N.D., Dugin, G.V., Pisarevsky, A.M., et al., Abstracts of Papers, Third International Congr. “Water: Ecology and Technology,” Moscow. 1998, pp. 341–342.Google Scholar
- JPN Appl. 09 311 130 A2.Google Scholar
- JPN Appl. 06 341 981 A2.Google Scholar
- Korenaga, T., Zhon, X., Okada, K., et al., Anal. Chim. Acta, 1993, vol. 272, no.2, pp. 237–244.Google Scholar
- Simal, J., Lage, M.A., and Iglesias, I., An. Bromatol., 1986, vol. 37, no.1, pp. 125–142.Google Scholar
- Zhang, S., Li, W., and Wang, H., Huagong Huanbao (China), 2001, vol. 21, no.3, pp. 171–173.Google Scholar
- Miller, D.G., Brayton, S.V., and Boyles, W.T., Water Environ. Res., 2001, vol. 73, no.1, pp. 63–71.PubMedGoogle Scholar
- Vaidya, B., Watson, S.W., Coldiron, S.J., et al., Anal. Chim. Acta, 1997, vol. 357, no.12, pp. 167–175.Google Scholar
- Lee, K.-H., Ishikawa, T., McNiven, S.J., et al., Anal. Chim. Acta, 1999, vol. 398, nos.2–3, pp. 161–171.Google Scholar
- Abdullin, I.F., Dement’enko, A.A., Budnikov, G.K., et al., Zavod. Lab., Diagnost. Mater., 2001, vol. 67, no.1, pp. 15–17.Google Scholar
- Chinese Patent 1 128 352 A.Google Scholar
- Chen, S.-Ch., Tzeng, J.-H., et al., Anal. Sci. (Taiwan), 2001, vol. 17, no.4, pp. 551–553.PubMedGoogle Scholar
- Rustioni, M., Cassani, G., Faccetti, E., Nucci, G., et al., in 4th World Surfactants Congr., Barcelona, 1996, vol. 1, pp. 474–488.Google Scholar
- Jardin, W.F. and Rohwedder, J.J., Water Res., 1989, vol. 23, no.8, pp. 1069–1071.Google Scholar
- Beltra, A.P., Iniesta, J., Gras, L., et al., Instr. Sci. Technol., 2003, vol. 31, no.3, pp. 249–259.Google Scholar
- Chen, H., An, T.-C., Fang, Y.-J., et al., Yankuany Ceshi (China), 1999, vol. 18, no.3, pp. 185–188.Google Scholar
- Vallejo-Pecharroman, B., Izquierdo-Reina, A., and Castro, L. de, Analyst, 1999, vol. 124, no.8., pp. 1261–1264.Google Scholar
- Schmitz, A., Eberhardt, R., Spohn, U., et al., DECHEMA Biotechnol. Conf., 1992, vol. 5, part B, pp. 1117–1120.Google Scholar
- FRG Patent 3 707 815 A1.Google Scholar
- Lee, K.-H., Ishikawa, T., Sasaki, S., et al., Electroanalysis, 1999, vol. 11, no.16, pp. 1172–1179.Google Scholar
- Vorontsov, A.M., Nikanorova, M.N., and Melent’ev, K.V., Abstracts of Papers, Vserossiiskaya konferentsiya “Sensor-2000” (Russian Conf. “Sensor-2000”), St. Petersburg, June 21–23, 2000, p. 21.Google Scholar
- Vorontsov, A.M., Nikanorova, M.N., and Melent’ev, K.V., Vodnye ob’ekty Sankt-Peterburga (Water Bodies of St. Petersburg), Kondrat’ev, S.A. and Frumin, G.T., Eds., St. Petersburg, 2002, pp. 73–78.Google Scholar
- Orhon, D. and Cokgor, E.U., J. Chem. Technol. Biotechnol., 1997, vol. 68, no.3, pp. 283–293.Google Scholar
- Vestner, R.I. and Gunthert, F.W., GWF, Wasser, Abwasser, 2001, vol. 142, no.9, pp. 635–644.Google Scholar
- Sperandio, M., Urbain, V., Ginestet, P., et al., Water Sci. Technol., 2001, vol. 43, part 1, pp. 181–190.Google Scholar
- Gorgun, E., Cokgor, E.U., Ohron, D., et al., Water Sci. Technol., 1995, vol. 32, no.12, pp. 43–52.Google Scholar
- Kato, Y., Kumagai, T., Nishioka, H., et al., Oriental J. Chem., 2001, vol. 17, no.1, pp. 1–8.Google Scholar
- Unmarino, G. and Russo, A., Mater. Conc. (Italy), 1996, vol. 72, no.1, pp. 51–52.Google Scholar
- Aisaka, K. and Mikashima, H., Examination of COD Testing Methods for Waters from Industrial Final Disposal Sites, Kenkyusho Nenpo (Japan), 1994, pp. 177–178.Google Scholar
- Bristol, P., Civiello, J., Harp, D., et al., Water Environment Federation Annual Conf., Atlanta, 2001, vol. 74, pp. 5436–5449.Google Scholar
- Belen’kaya, S.L., Popova, Yu.I., and Kalitvyanskaya, V.I., Ferment. Spirt. Prom-st., 1987, no. 4, pp. 13–14.Google Scholar
- Amarasinghe, H., Anusha, U., Gunavardena, H.D., et al., J. Nat. Sci. Council Sri Lanka, 1993, vol. 21, no.2, pp. 259–266.Google Scholar